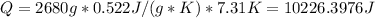Answer:
The energy needed is:
4447.1116 J for tin.
8777.906J for nickel.
10226.3976J for titanium.
Step-by-step explanation:
The equation you have to use to find the energy needed is :
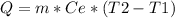
where m is the mass in grams, Ce is the specific heat of the substance, T2 is the final temperature in kelvin and T1 is the initial temperature in kelvin.
so to use the equation we have to change the mass to grams:
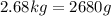
and we might change the temperature to kelvin but since the temperature difference is the same in C° than in K° we don't have to do that and we could use the temperature difference in C° which is the same in K°, so:
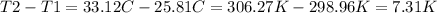
now we just have to repace in the equation for every material, lets do that:
for Tin:
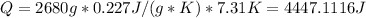
for Nickel:
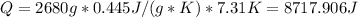
and for Titanium: