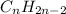Answer:
- The general molecular formula for cycloalkanes with two rings is:
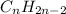
Step-by-step explanation:
As stated in the question statement, the general formula for an alkane is:
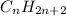
That is because each of the two carbons of the ends are bonded to three hydrogen atoms and the other carbons are bonded to two atoms:
This example shows you how that works.
Assume a four carbon linear chain, i.e. n = 4:
- Two carbons of the ends ↔ six hydrogens
- Two internal carbons ↔ four hydrogens
- 2 + 2 = 4 carbons ↔ 6 + 4 = 10 hydrogens
- 4 ↔ 2×4 + 2
For an alkane of one ring or with one ring, the general formula is:
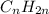
That is because there not ending carbons: every carbon atom is attached to other carbon atom and two hydrogen atoms.
If you add a second ring, the molecule will have two less hydrogen atoms relative to the alkance with one ring, because you are adding one extra carbon - carbon bond, reducing the number of hydrogen atoms attached in two.
Thus, the general formula for cycloalkanes with two rings is: