Answer:
t = 2.6 billion years
Step-by-step explanation:
If potassium becomes 25% of its initial value
so we can say it becomes half two times
as we know that 25% means it is 1/4 times of initial value
so we will have
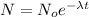
here we know that

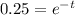
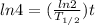
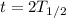
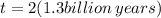
t = 2.6 billion years