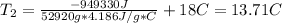Answer:
Final temperature 13.71 C the plan won't work
Step-by-step explanation:
Reaction:
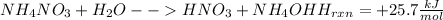
Mol of
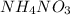
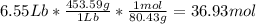
Mol of water
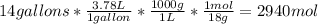
The reaction stoichiometry is 1:1 so the
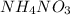 with 36.93 mol is the limit reagent in the reaction.
with 36.93 mol is the limit reagent in the reaction.
The energy for this reaction is:
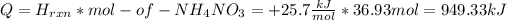
The process is getting this energy from the water for that reason the temperature of the water will decrease.
Q necessary for the reaction is
[
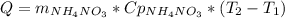
The heat given by the water will be the same but with a different sign
[
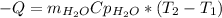
We estimate the water’s final temperature, with this equation:
Clearing for T2 we get:
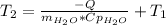
mass of water =52920g
Cp=4.186 J/g*C