Answer:
final pressure is = 425 kPa
Step-by-step explanation:
Allowing ideal gas to expand in whole tank, therefore resisiting force in this process is zero. thus work done si also zero. considering ideal gas as system , there is no heat transefer during process of expansion.
Assuming : the gas act like ideal gas, change in kinetic and potential energy is negligible
from 1 st law we have
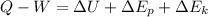
By taking consideration of above assumption we get
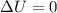
i.e.
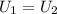
from ideal gas equation we hvae
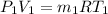
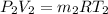
as internal energy remain same in both state therefore temperature remain same
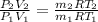
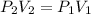
as we knwo that
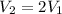
therefore we have
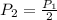
= 425 kPa