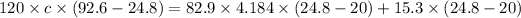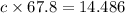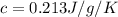Answer:c=0.213 J/g/K
Step-by-step explanation:
Given
sample mass
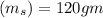
initial temperature of mineral
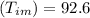
mass of water
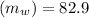
Water initial temperature
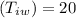
Heat capacity of calorimeter =15.3 J/k
Final Temperature is 24.8
let c be the specific heat of mineral
Heat released by mineral sample=heat absorbed by calorimeter and water