Answer:
Step-by-step explanation:
Given data:
initial construction co = 0.286 wt %
concentration at surface position cs = 0 wt %
carbon concentration cx = 0.215 wt%
time = 7 hr
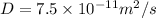
for 0.225% carbon concentration following formula is used
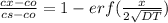
where, erf stand for error function
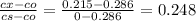
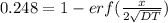
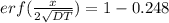
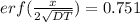
from the table erf(Z) value = 0.751 lie between (z) = 0.80 and z = 0.85 so by inteerpolation we have z = 0.815
from given table
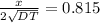
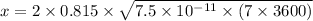
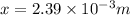
x = 0.002395 mm