Answer:
a) Mean = 4
b) Standard deviation = 0.9308 ; Variance = 0.8663
c) attached in the file
Explanation:
Given:
4.2, 4.7, 4.7, 5.0, 3.8, 3.6, 3.0, 5.1, 3.1, 3.8, 4.8, 4.0, 5.2, 4.3, 2.8, 2.0, 2.8, 3.3, 4.8, and 5.0
n = 20
a) Mean =
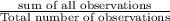
thus,
Mean =
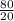
or
Mean = 4
b)
data data-mean (data - mean)²
4.2 0.2 0.04
4.7 0.7 0.49
4.7 0.7 0.49
5.0 1 1
3.8 -0.2 0.04
3.6 -0.4 0.16
3.0 -1 1
5.1 1.1 1.21
3.1 -0.9 0.81
3.8 -0.2 0.04
4.8 0.8 0.64
4.0 0 0
5.2 1.2 1.44
4.3 0.3 0.09
2.8 -1.2 1.44
2.0 -2 4
2.8 -1.2 1.44
3.3 -0.7 0.49
4.8 0.8 0.64
5.0 1 1
====================================
Now, ∑(Data - Mean)² = 16.46
and,
Standard deviation =
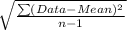
or
Standard deviation =
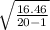
or
Standard deviation = 0.9308
variance = ( Standard deviation )²
or
Variance = 0.9308² = 0.8663
c) the plot is attached in the file