Answer:
49,736.84 kJ
Step-by-step explanation:
1 L of gasoline has 0.70 kg
1,5 L of gasoline has x kg
1 L - 0.70 kg
1.5 - x
x = 0.7 *1.5 = 1,05 kg
1 mol of gasoline has 114 g
y mol of gasoline has 1050 g
1 mol - 114 g
y - 1050 g
y =
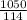 = 9.21 mol
= 9.21 mol
1 mol of gasoline has a combustion heat of 5400 kJ
9.21 mol of gasoline has a combustion heat of z
z = 5400 * 9.21 = 49,736.84 kJ