Answer:
The new volume is 6 L.
Step-by-step explanation:
Boyle’s law states that,
For a given amount of a gas, Pressure is inversely proportional to its volume at constant temperature
Thus the expression is PV = constant (T and n are constant)
When there is a change in pressure and volume, we make use of the expression
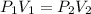 (T and n are constants)
(T and n are constants)
Where
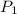 and
and
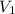 are the Initial pressure and volume and
are the Initial pressure and volume and
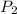 and
and
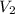 are the final pressure and volume
are the final pressure and volume
Inversely proportional means when one increases the other one decreases. That is as per Boyle’s law when Pressure increases the volume will decrease and vice versa.
Let us solve this problem now
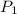 = 3atm
= 3atm
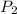 = 4atm
= 4atm
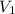 = 8L
= 8L
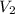 = ?
= ?
Plugging in the values
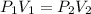 (T and n are constants)
(T and n are constants)
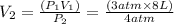
= 6 L is the Answer