Answer: Option (C) is the correct answer.
Step-by-step explanation:
A neutral hydrogen atom is defined as the atom in which electrons are resent in their ground state only.
Basically in a neutral atom electrons are present in their parent shell.
For example, atomic number of hydrogen is 1 and electronic configuration of a neutral atom of hydrogen is
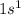 .
.
Hence, it means there is only one electron present in its valence shell. So, in order to attain stability it will readily react with another atom to gain or share an electron.
Thus, we can conclude that the statement it is less chemically reactive than an atom of aluminum (Al), is false.