Answer:
25.05533 amu
Step-by-step explanation:
The formula for the calculation of the average atomic mass is:
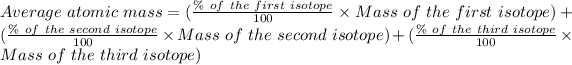
The % of Mg 24 = 78.99 %
Let the % of Mg 26 = x %
The % of Mg 25 = 0.9083 x%
Thus, 78.99 + x + 0.9083 x = 100
x = 11.01 %
% of Mg 25 = 10 %
Given that:
For first isotope:
% = 78.99 %
Mass = 23.98504 amu
For second isotope:
% = 11.01 %
Mass = 25.98259 amu
For third isotope:
% = 10 %
Mass = A amu
Average mass = 24.312 amu
Thus,
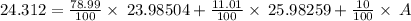
A = 25.05533 amu