Answer:
The given statement is false.
Step-by-step explanation:
As per Avogadro’s law under constant temperature and pressure there will be same amount of moles present in the same volume of gas. If the gas increases, the amount of moles also increases. In other words we can say that the volume of gas(V) is directly proportional to the number of moles present (n). So we can say,
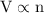
The amount of moles is called Avogadro’s constant.