Answer: The balanced chemical equation is written below.
Step-by-step explanation:
There are 2 types of chemical reaction classified on the basis of heat change:
- Endothermic reactions : They are the reactions in which energy of products is more than the energy of the reactants. For these reactions, energy is absorbed by the system. The
 comes out to be positive and is written on the reactants side.
comes out to be positive and is written on the reactants side. - Exothermic reactions: They are the reactions in which energy of reactants is more than the energy of the products. For these reactions, energy is released by the system. The
 comes out to be negative and is written on the product side.
comes out to be negative and is written on the product side.
We are given:
Moles of methanol = 2 moles
Moles of methane = 2 moles
Moles of oxygen gas = 1 mole
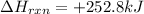
The chemical equation follows:
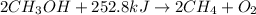
Hence, the balanced chemical equation is written above.