Answer: The mass percent of nitrogen gas in the compound is 13.3 %
Step-by-step explanation:
Assuming the chemical equation of the compound forming product gases is:
![\text{Compound}\xrightarrow[CuO(s)]{Hot}N_2(g)+CO_2(g)+H_2O(g)](https://img.qammunity.org/2020/formulas/chemistry/high-school/6yz19svgvmvz2u5mio498tet32rdy4igtl.png)
Now, the product gases are treated with KOH to remove carbon dioxide.
We are given:
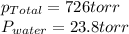
So, pressure of nitrogen gas will be =
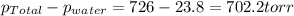
To calculate the number of moles of nitrogen, we use the equation given by ideal gas which follows:

where,
P = pressure of nitrogen gas = 702.2 torr = 0.924 atm (Conversion factor: 1 atm = 760 torr)
V = Volume of nitrogen gas = 31.8 mL = 0.0318 L (Conversion factor: 1 L = 1000 mL)
T = Temperature of nitrogen gas =
![25^oC=[25+273]K=298K](https://img.qammunity.org/2020/formulas/physics/high-school/h3swi627jfkpg7vx7in8p5pe35bz1gwehq.png)
R = Gas constant =
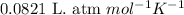
n = number of moles of nitrogen gas = ?
Putting values in above equation, we get:
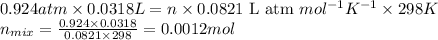
- To calculate the mass of nitrogen gas, we use the equation:

Molar mass of nitrogen gas = 28 g/mol
Moles of nitrogen gas = 0.0012 moles
Putting values in above equation, we get:

- To calculate the mass percent of nitrogen gas in compound, we use the equation:
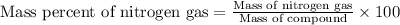
Mass of compound = 0.253 g
Mass of nitrogen gas = 0.0336 g
Putting values in above equation, we get:
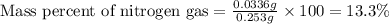
Hence, the mass percent of nitrogen gas in the compound is 13.3 %