Answer:
Equilibrium concentration of Br₂ = 0.02 M
Step-by-step explanation:
Moles of hydrogen gas :
Given, Mass of H₂ = 1.374 g
Molar mass of H₂ = 2.016 g/mol
The formula for the calculation of moles is shown below:
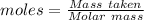
Thus,
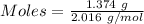
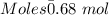
Moles of Bromine gas :
Given, Mass of Br₂ = 70.31 g
Molar mass of Br₂ = 159.808 g/mol
The formula for the calculation of moles is shown below:
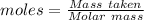
Thus,
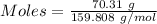
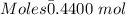
Considering the ICE table for the equilibrium as:
H₂(g) + Br₂(g) ⇌ 2HBr(g)
t = o 0.68 0.44 0
t = eq -x -x +2x
--------------------------------------------- -----------------------------
Moles at eq: 0.68-x 0.44-x 2x
Given that: At equilibrium the vessel is found to contain 0.566 g of H₂
Moles = 0.566 g / 2.016 g/mol = 0.28 moles
Thus, 0.68 - x = 0.28
x = 0.40 moles
Volume = 2.00 L
Equilibrium moles of Br₂ = 0.44 - 0.40 moles = 0.04 moles
Equilibrium concentration of Br₂ = 0.04 moles/ 2 L = 0.02 M