Answer:
iron = 15000 kg 95% sulfuric acid = 27646.32 kg
Step-by-step explanation:
The molar volume to a gas is 22.4 L, which means that a mole of a given gas at 0°C and 1 atm will have a volume os 22.4 L.
1 m3 - 1000 L
4870 m3 - x
x = 4870 * 1000 = 4.87 *10^6 L
1 mole - 22.4 L
y mole - 4.87 * 10^6 L
y =
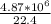 = 2,17 *10^5 moles (if 100% of the H2 is used)
= 2,17 *10^5 moles (if 100% of the H2 is used)
as 20% of it is lost, we need to know how much more we need to use.
80% - 2.17 * 10^5 moles
100% - z
z =
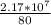 = 2,68*10^5 moles
= 2,68*10^5 moles
so we would need 2.68*10^5 moles of iron and sulfuric acid.
1 mole of iron - 56 *10^-3 kg
2.68*10^5 moles - t
t= 2.68*56 *10^2 = 15000 kg
1 mole of sulfuric acid - 98 * 10^-3 kg
2.68*10^5 - u
u = 2.68*98 *10^2 = 26264 kg (if 100%)
95% - 26264 kg
100% - v
v =
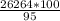 = 27646.32 kg
= 27646.32 kg