Answer:
C) [PCl5] = 0.0280 M, [PCl3] = 0.222 M, [Cl2] = 0.222 M
Step-by-step explanation:
Moles of [PCl₅] = 0.250 M
Considering the ICE table for the equilibrium as:
PCl₅ (g) ⇔ PCl₃ (g) + Cl₂ (g)
t = o 0.250
t = eq -x x x
--------------------------------------------- --------------------------
Moles at eq: 0.250-x x x
The expression for the equilibrium constant is:
![K_c=\frac {[PCl_3][Cl_2]}{[PCl_5]}=1.80](https://img.qammunity.org/2020/formulas/chemistry/college/n01kuuuud2wtdo8lsxcgb8y5o21a3qwhz3.png)
So,
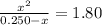
x = 0.222 M
Equilibrium concentrations :
[PCl₃] = [Cl₂] = 0.222 M
[PCl₅] = 0.250 - 0.222 = 0.280 M
Option C is correct.