Answer: The mass fraction of
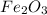 in the original sample is 0.637
in the original sample is 0.637
Step-by-step explanation:
We are given:
Mass of sample = 3.109 g
Mass of solid residue (Aluminium oxide + iron) = 2.515 g
Amount of mass lost = (3.109 - 2.515) = 0.594 g
The amount of mass lost from the sample is equal to the mass of oxygen lost from iron (III) oxide.
The chemical equation for the reaction of iron (III) oxide with hydrogen follows:
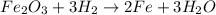
In 1 mole of iron (III) oxide, 2 moles of iron are present and 3 moles of oxygen are present.
Mass of oxygen lost from 1 mole of iron (III) oxide = (3 × 16) = 48 g
Calculating the mass of iron (III) oxide by using unitary method:
48 grams of oxygen is lost when 159.69 grams of iron (III) oxide is reacted with hydrogen gas
So, 0.594 grams of oxygen will be lost when =
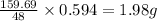 of iron (III) oxide is reacted with hydrogen gas
of iron (III) oxide is reacted with hydrogen gas
- To calculate the mass fraction of iron (III) oxide, we use the equation:
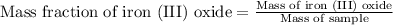
Mass of iron (III) oxide = 1.98 grams
Mass of sample = 3.109 grams
Putting values in above equation, we get:
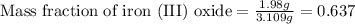
Hence, the mass fraction of
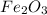 in the original sample is 0.637
in the original sample is 0.637