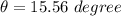Answer:
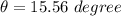
Step-by-step explanation:
Heat required to heat ice is ΔQ1
ΔQ1 = msΔQ
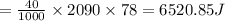
Heat required to melt ice is ΔQ2
ΔQ2 = mL
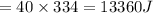
Heat required to coll to o degree c is ΔQ3
ΔQ3
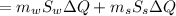
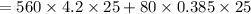
ΔQ3 = 59570 J
since ΔQ3 > ΔQ1 + ΔQ2
New temperature \theta at which ice melt to water
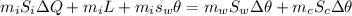
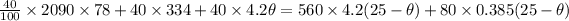
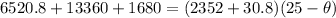
solving for
 we get
we get