Answer:
Bases turns red litmus paper blue.
Step-by-step explanation:
Arrhenius theory states a Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples
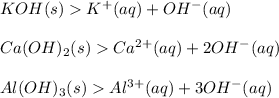
Please note: (aq) stands for aqueous which means in the presence of water that is, water acts as a solvent.
Bases consists of a cation and hydroxide as its anion in the above examples.
Arrhenius Theory: An acid is a substance which produces one or more hydrogen ions, (H+) in aqueous solution.
Examples:
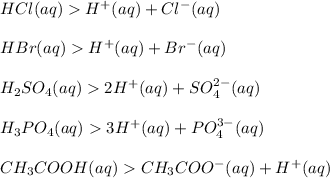
Bronsted Lowry Theory:
An acid is a substance that can donate one or more proton
A base is a substance which can accept one or more protons
HA (acid) loses H+ to form A-
B (base) gains H+ to form HB+