Answer:
A)
Solution:
Adding a strong acid such as HCl to water results in the release of H+ ions.
Arrhenius Theory: An acid is a substance which produces one or more hydrogen ions, (H+) in aqueous solution.
Examples:
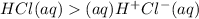
(H+) from the acid binds to neutral water molecules to form
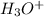 raising the concentration of H+.
raising the concentration of H+.
The resulting large concentration of (H+) makes the solution more acidic and leads to a dramatic drop in the pH.
B)
Solution :
An acid is a substance which produces one or more hydrogen ions, (H+) in aqueous solution.
Examples:
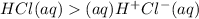
So, HCl is an acid.
C)
Solution :
A Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples :
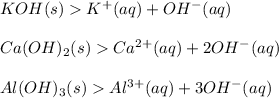
Please note: (aq) stands for aqueous which means in the presence of water that is, water acts as a solvent
The release of OH- ions makes the solution more basic and this leads to an increase in pH of the solution
D)
Solution:
A Base is a substance which produces one or more hydroxyl ion or hydroxide ion (OH-) in aqueous solution.
Examples
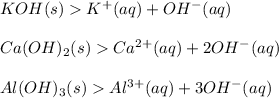
Please note: (aq) stands for aqueous which means in the presence of water that is, water acts as a solvent
So, NaOH is a Base.