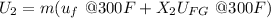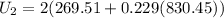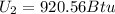Answer:
a) pressure = 66.985 psia
b) internal energy 920.56 Btu
Step-by-step explanation:
Given data:
left chamber characterisitics
2lbm water, 500 psia, 1.5 ft^3
right chamber
volume = 1.5 ft^3
when partitioned ruptured, the final volume will be
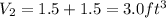
specific volume will be
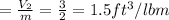
at
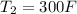 , From saturated water tables
, From saturated water tables
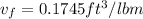
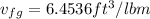

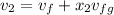
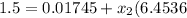
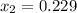
the final pressure at temperature 300 F
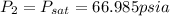
internal energy at fnal stage