Answer:
correct option is C i.e F_{fes} [F] > F_{fes} [He]
Step-by-step explanation:
Given data:
excited state of helium is given as 159,850 cm^{-1}
In terms of joule =
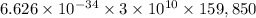

First excited state of fluorine is
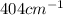
in terms of joule is
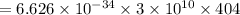

so,
![F_(fes) [He] = e^{- \frac {3.177* 10^(-18)}{1.38* 10^(-23)* 1000}](https://img.qammunity.org/2020/formulas/chemistry/high-school/hjwozgyte4tdhx039xol7qb8rkp7dennj4.png)
where T =1000 k
![F_(fes) [He] = 0](https://img.qammunity.org/2020/formulas/chemistry/high-school/rgq9nwnubwoczu6bkzrv53m2xxj3e9akzu.png)
![F_(fes) [He] = e^{- \frac {8.030* 10^(-21)}{1.38* 10^(-23)* 1000}](https://img.qammunity.org/2020/formulas/chemistry/high-school/hi4jyilfnm4axkb97rqdlp6lhuwzxhq4hu.png)
where T = 1000 k
![F_(fes) [F] = 0.55](https://img.qammunity.org/2020/formulas/chemistry/high-school/o8n7ndf92zc66slqxpce8kzxsbfsscfy82.png)
Therefore .
![F_(fes) [F] > F_(fes) [He]](https://img.qammunity.org/2020/formulas/chemistry/high-school/auljyi5nx3w2qixapuykp7gtczgoexqull.png)
correct option is C