Answer:
option A.
Step-by-step explanation:
given,
The abundance and atomic mass of In-113 is 4.29 % and 112.904
The abundance and atomic mass of In-115 is 95.71 % and 114.904
Average atomic mass =
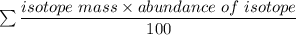
Average atomic mass =
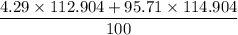
=
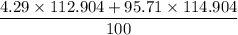
= 114.818
the average atomic mass comes out to be 114.818
hence, the correct answer is option A.