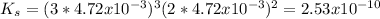Answer:
The Ksp of the magnesium phosphate is 2.53x10⁻¹⁰
Step-by-step explanation:
The molar mass of magnesium phosphate is:
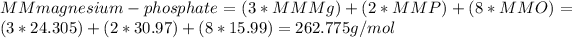
The number of moles is:
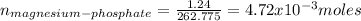
The molarity is:
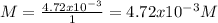
The dissociation of magnesium phosphate is:
Mg₃(PO₄)₂ = 3Mg²⁺ + 2PO₄³⁻
The Ks is:
![K_(s) =[Mg^(2+)]^(3)[PO_(4)^(3-) ]^(2) =(3s)^(3) (2s)^(2)](https://img.qammunity.org/2020/formulas/chemistry/high-school/in55kzomy9u7o1u31fbeatmmv6jjnu6hq9.png)
Where
s = solubility of ions = 4.72x10⁻³
Replacing