Answer:
3)The reaction is not at equilibrium and willproceed to the right.
Step-by-step explanation:
The reaction quotient of an equilibrium reaction measures relative amounts of the products and the reactants present during the course of the reaction at particular point in the time.
It is the ratio of the concentration of the products and the reactants each raised to their stoichiometric coefficients. The concentration of the liquid and the gaseous species does not change and thus is not written in the expression.
Q < Kc , reaction will proceed in forward direction.
Q > Kc , reaction will proceed in backward direction.
Q = Kc , reaction at equilibrium.
Given that:
Q =
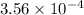
K =
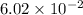
Since, Q < K , reaction is not at equilibrium and will proceed to right, in forward direction.