Answer:
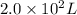 liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level
liters of fluoridated drinking water would a 70−kg person have to consume in one day to reach this toxic level
Step-by-step explanation:
1g =1000mg
So,
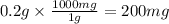 is toxic for 70kg person
is toxic for 70kg person
Fluoride present in drinking water is 1mg per L.
So 200mg is present in 200L
200L of fluoridated drinking water is toxic to a 70kg person which can cause death.
Using dimensional analysis,
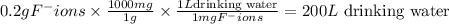
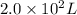 drinking water (Answer)
drinking water (Answer)