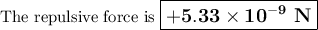Answer:
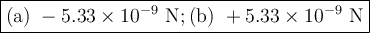
Step-by-step explanation:
a) Attractive force
To calculate the attractive force (F), we can use Coulomb's Law:
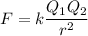
where
Q₁ and Q₂ are the charges on the ions,
r is the distance between them, and
k = the Coulomb constant
Data:
Q₁ = 1+
Q₂ = 1-
r₊ = 0.068 nm
r₋ = 0.140 nm
k = 8.988 × 10⁹ N· m²C⁻²
Calculations:
Q₁ = (+1) × 1.602 × 10⁻¹⁹ C = +1.602 × 10⁻¹⁹ C
Q₂ = (-1) × 1.602 × 10⁻¹⁹ C = -1.602 × 10⁻¹⁹ C
r = r₊ + r₋ = 0.068 nm + 0.140 nm = 0.208 nm = 0.208 × 10⁻⁹ m
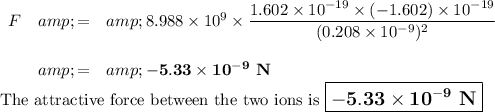
The negative sign shows that the ions are attracted to each other.
(b) Repulsive force
The equilibrium position is reached when the ions just touch each other. If they come any closer, the nuclear repulsions will outweigh the Coulombic attraction.
Thus, the repulsive force is equal and opposite to the attractive force.