Answer:
Δ
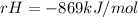
Step-by-step explanation:
Hello, for this chemical reaction, the overall enthalpy of reaction is defined by:
Δ
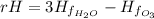
Since the enthalpy of formation of water is 0 as long as it is a pure element. Now, applying the formula with the proper values (extracted from the NIST database), we get:
Δ
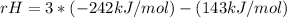
Δ
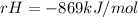
Best regards.