Answer:
Step-by-step explanation:
Hello,
Based on the formula:
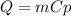 Δ
Δ

By assuming that both the mass and the temperature difference is equal for all the metals, the most amount of heat loss will be experienced by the aluminium since it has the highest heat capacity (more released or absorbed energy per unit of mass by degree Celsius).
Best regards.