Answer:
Yes, Al change oxidation number
Step-by-step explanation:
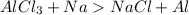
For a free element, oxidation number is 0.
Na and Al are free elements we find in the equation as they are not in combination with other elements.
For a compound, the sum of oxidation number of elements = 0.
We know oxidation number of chlorine is -1.
So let us find the oxidation number of Al in
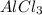
Let x be the oxidation number of Al so,
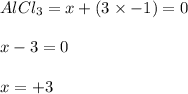
Oxidation number of Al in
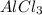 is +3
is +3
Oxidation number of Al changes from +3 to 0.
Decrease in oxidation number indicates
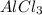 is getting reduced in the reaction.
is getting reduced in the reaction.
Na oxidation number increases from 0 to +1 shows Na is getting oxidised in the reaction
The change in oxidation number of the elements represents a redox reaction where both oxidation and reduction takes place simultaneously.