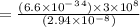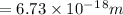Answer:
1. The frequency of green light that has a wavelength of the
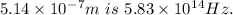
2. Wavelength of infrared radiation with frequency
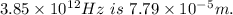
3. Energy of a photon with frequency
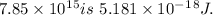
4. The energy of a photon having a wavelength of
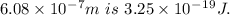
5. Wavelength of ultraviolet radiation having
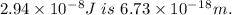
Step-by-step explanation:
The equation connecting wavelength, frequency and speed of electromagnetic radiation is
c=ϑλ
1. λ =
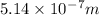
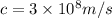
ϑ = c/λ =
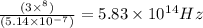
2. ϑ =
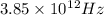
λ= c/ϑ
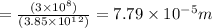
3. E = h ϑ
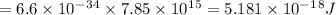
4. E= hc/λ
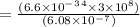
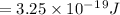
5. E=
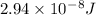
λ= hc/E