Answer:
2.5 M
Step-by-step explanation:
To address this question, we can use the dilution formula:
M1V1= M2V2
Where M1 is the concentration of the concentrated solution
V1 is the volume of the concentrated solution (the amount of mL that we take from the initial solution)
M2 is the concentration of the diluted solution
V2 is the volume of the diluted solution or the final volume.
For this case, we know V1, M2 and V2 and we want to know M1.
Following the formula, M1=
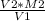
Therefore, M1 =
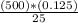
For this, the concentration of the original solution is 2.5 M.
I hope this clarifies your inquiry.