Answer:
1) ∆H is positive
 Endothermic
Endothermic
2)

 Endothermic
Endothermic
3) Energy is absorbed
 Endothermic
Endothermic
4)
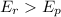
 Exothermic
Exothermic
5) ∆H is negtive
 Exothermic
Exothermic
Step-by-step explanation:
∆H is called as enthalpy change
It is also called as Heat of reaction
Energy is required for the bond to break a bond.
Energy is released when a bond is formed.
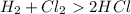
that is
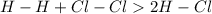
We see in this equation, bonds between hydrogen and chlorine molecules gets broken and on the right side bond is formed in HCl.
If energy of products greater than energy of reactants then the reaction enthalpy change is endothermic .
If energy of products lesser than energy of reactants then the reaction enthalpy change is exothermic .
For example
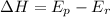
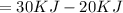
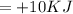
(positive hence endothermic)
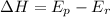
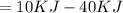
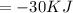
(negative hence exothermic)