Answer : The correct option is, It would increases.
Explanation :
Boyle's Law : It is defined as the pressure of the gas is inversely proportional to the volume of the gas at constant temperature and number of moles.
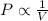
From this we conclude that, the pressure is inversely proportional to the volume that means, as the pressure increases the volume will be decreases and pressure decreases the volume increases.
As per question, when the gas is moved from a large container to a small container then the pressure of the gas increases.
Hence, the correct option is, It would increases.