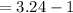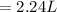Answer:
To raise the pH of the solution to 3.10 we have to add 2.34 L of water.
Step-by-step explanation:
Given that the pH of the solution of HCl in water is 2.5. Here the solution’s pH is changing from 2.5 to 3.10 which means the acidic nature of the solution is decreasing here on dilution.
![[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/jptxicd74sqji6oqiawrnboqrtetqtax3v.png) ions contribute to a solution’s acidic nature and
ions contribute to a solution’s acidic nature and
![[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/xxde7ud270ullhgkdtd9pogar35adf8qg0.png) contribute to a solution’s basic nature.
contribute to a solution’s basic nature.
The equation connecting the concentration of
![[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/jptxicd74sqji6oqiawrnboqrtetqtax3v.png) and pH of a solution is pH=
and pH of a solution is pH=
![-log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/37dhyjhb8h5dh5um4wd2h6ot4ln6aatikr.png)
![[H^+]= 10^(^-^p^H^)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/mchj19ea357xf5oycvkipmauli9orop3jz.png)
![[H^+]= 10^(^-^2^.^5^)=0.00316](https://img.qammunity.org/2020/formulas/chemistry/middle-school/tywxvtlliqosyrs5rp74bn4swg3rmgfz58.png)
When the pH is
![3.1 [H^+ ]= 10^(^-^3^.^1^)=0.000794](https://img.qammunity.org/2020/formulas/chemistry/middle-school/w8ph6v37ekv45py3tqymd5eu87no03hw9p.png)
On dilution the concentration of a solution decreases and volume increases.
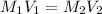
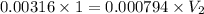
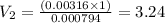
Volume of water to be added