Answer:
A. uniform
Step-by-step explanation:
We can solve this problem by using Boyle's law, which states that:
"For a constant mass of an ideal gas kept at constant temperature, the pressure of the gas is inversely proportional to its volume".
Mathematically:
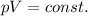
where
p is the pressure of the gas
V is its volume
If we apply the equation to the bottle in the problem, we see that:
- when the volume of the bottle (and therefore, of the gas inside) decreases, than the pressure will increase
- viceversa, when the volume of the bottle increases, the pressure will decrease
The amount by which the pressure increases is inversely proportional to the decrease in volume, so the answer depends on how the volume of the bottle decreases: however, if the volume of the bottle decreases uniformly, then we can say that the pressure inside the bottle will also increases uniformly.