Answer:
113.6 ml of methanol is used to make 355 ml of a 32.0
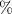 V/v solution in water.
V/v solution in water.
Step-by-step explanation:
A 32
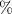 V/v solution of methanol contains 32 ml of methanol in 100 ml of solution. So, volume of methanol needed to make 355 mL of a 32
V/v solution of methanol contains 32 ml of methanol in 100 ml of solution. So, volume of methanol needed to make 355 mL of a 32
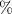 V/v solution is
V/v solution is
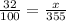
On solving,
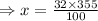
 x=113.6 ml
x=113.6 ml