Answer:
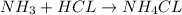 is a combination reaction.
is a combination reaction.
Step-by-step explanation:
THE REACTION BETWEEN
 AND HCL TO FORM
AND HCL TO FORM
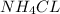 IS A COMBINATION REACTION.
IS A COMBINATION REACTION.
Here the compound ammonia is reacted with the acid hydrochloric acid to form a salt ammonium chloride.
When two or more elements or compounds or mixture of elements and compounds are combined to form a single compound, it is called a combination reaction.
Here,the two compounds are combined to form single compound
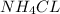 .
.