Answer:
1. 266.22 g/mol
2. 168.81 g/mol
3. 223.35 g/mol
4. 199.88 g/mol
Step-by-step explanation:
For you to calculate the molar mass of the salt you need to sum the molar masses of every element in the salt.
In the first salt, PdBr
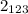 , the subscript 2 means that there are 2 atoms of Br. So for you to calculate the molar mass of the salt you need to sum the molar mass of Pd and 2 times the molar mass of Br, as follows:
, the subscript 2 means that there are 2 atoms of Br. So for you to calculate the molar mass of the salt you need to sum the molar mass of Pd and 2 times the molar mass of Br, as follows:
106 g/mol + 2(79.90 g/mol) = 266.22 g/mol
In the second salt BeBr
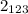 there are 2 atoms of Br and 1 of Be, so the molar mass is:
there are 2 atoms of Br and 1 of Be, so the molar mass is:
9.012 g/mol +2(79.90 g/mol) = 186.22 g/mol
In the third salt CuBr
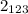 there are 2 atoms of Br and 1 of Cu, so the molar mass is:
there are 2 atoms of Br and 1 of Cu, so the molar mass is:
63.55 g/mol + 2(79.90 g/mol) = 223.35 g/mol
And in the fourth salt CaBr
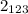 there are 2 atoms of Br and 1 of Ca, so the molar mass is:
there are 2 atoms of Br and 1 of Ca, so the molar mass is:
40.08 g/mol + 2(79.90 g/mol) = 199.88 g/mol