Answer:
The molecular formula is C₅H₅OBr
Solution and explanation:
The molar mass of the compound is 161 g/mol
Elements contained in the compound are carbon, hydrogen, bromine, and oxygen.
Atomic masses are;
Oxygen - 16.0 g/mol
Bromine - 79.90 g/mol
Carbon - 12.01 g/mol
Hydrogen - 1.008 g/mol
Assuming the compound contains 1 mole of Bromine and Oxygen
Therefore;
Total mass of bromine and oxygen in the compound= 16.0 g/mol + 79.90 g/mol
If there are x atoms of hydrogen in the compound, then;
Mass of Hydrogen = 1.008 x
Carbon is 12 times the mass of hydrogen, thus
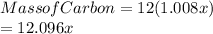
Hence;
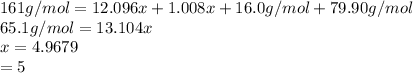
Therefore;
Hydrogen atoms = 5
Carbon atoms= 5(12.096)/12.01
= 5
Bromine atoms = 1
Oxygen atom = 1
Therefore;
The molecular formula is C₅H₅OBr