Answer:
The answer to your question is: 2 grams of methane
Step-by-step explanation:
Data
V = 9.15 l
P = 1.77 atm
T = 57° C = 330 °K
Ar mass = 19 g
CH4 mass = ?
Formula
PV = nRT
Process
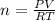
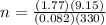
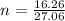
n = 0.6
Argon
40 g of Ar -------------------- 1 mol
19 g --------------------- x
x = 0.475 mol of Ar
moles of CH4 = 0.6 - 0.475
= 0.125
Methane
16 g of CH4 --------------- 1mol
x ---------------- 0.125 mol
x = 2 grams