For this case we can translate the given statement as:
Isopropyl alcohol vapors have been sampled using an activated carbon tube for 8 hours at a flow of 20 ml/min: what was the volume of the sampled air?
By definition, the volumetric flow rate is given by:
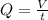
Where:
V: It's the volume
t: It's time
Then, the volume is given by:
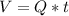
According to the problem data we have:
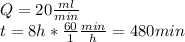
Substituting the values we have:
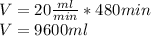
Finally, the volume of the sample is:
9600ml
Answer:
9600ml