Answer:
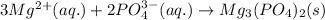
Step-by-step explanation:
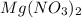 ,
,
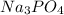 are strong electrolytes. Hence they are fully ionized in aqueous solution.
are strong electrolytes. Hence they are fully ionized in aqueous solution.
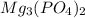 is a sparingly soluble salt. Hence it remains undissociated in aqueous solution.
is a sparingly soluble salt. Hence it remains undissociated in aqueous solution.
So, total ionic equation:
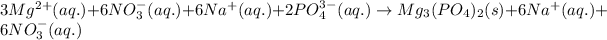
Net ionic equation is written by omitting spectator ions from total ionic equation.
Here,
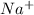 and
and
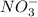 ions are spectator ions as they remain present on both side of total ionic equation.
ions are spectator ions as they remain present on both side of total ionic equation.
So, net ionic equation:
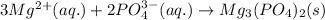
So, option (d) is correct.