Answer:
Mass of copper metal will be 2.06756 gram
Step-by-step explanation:
We have given number of moles of copper n = 0.03256
Molar mass of copper = 63.5 u
We have to calculate mass of copper metals in gram
We know that number of moles is given by
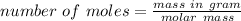
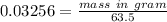
Mass in gram = 2.06756 gram
So mass of copper metal will be 2.06756 gram