Answer:
3.49X10⁻¹⁷ J
Step-by-step explanation:
The energy of an electron is given by the equation:
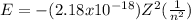
Where Z is the number of protons of the atom and n is the energy level of the electron. For Be, Z = 4.
When n tends to infinity (1/n²) tends to 0, and at this point, the electron has left the atom, so it has ionized.
The ionization energy then is the energy of the electron that left the atom less the energy of the electron in the energy level:
I.E = - (2.18x10⁻¹⁸)x4²x0 - (-(2.18x10⁻¹⁸)x4²x(1/1²))
I.E = 3.49X10⁻¹⁷ J