Answer:C) an atom must have a positively charged center that contains most of its mass.
Step-by-step explanation:
Rutherford model, also called nuclear atom or planetary model of the atom, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford.
During the experiment it was noticed that a few of the positively charged alpha particles got deflected back and some deviated away at an angle.
- The model described the atom as a tiny, dense, positively charged core called a nucleus, in which nearly all the mass is concentrated because the protons and the neutrons reside in it, around which the light, negative constituents, called electrons, circulate at some distance, much like planets revolving around the Sun.
- Mass of an electron is 9.10938356 ×
 kilograms.
kilograms. - Mass of a proton is 1.6726219 ×
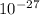 kilograms.
kilograms.
- Mass of a neutron is 1.674927471×
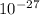 kilograms which slightly heavier than a proton.
kilograms which slightly heavier than a proton.
The nucleus is positively charged because it contains protons which are positively charged and the neutrons which have no charge, hence most of its mass is concentrated in the nucleus.