Answer: The insoluble substance will be AZ and BY
Step-by-step explanation:
Precipitation reaction is defined as the reaction in which an insoluble salt is formed when two solutions are mixed containing soluble substances. The insoluble salt settles down at the bottom of the reaction mixture.
Double displacement reaction is defined as the reaction in which exchange of ions takes place.
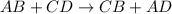
For a reaction to form precipitate, the ions must be exchanged in the reaction.
For the given chemical equations:
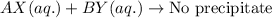
No exchange of ions took place in the above reaction.
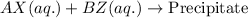
As, precipitate is forming in the above reaction, the exchange of ions took place.
The reaction now becomes:
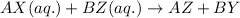
Hence, the insoluble substance will be AZ and BY