Answer:
The answer to your question is: P2 = 772.35 torr.
Step-by-step explanation:
Data
P1 = 3990 torr.
V1 = 3.41 L
T1 = -91.5°C = 181.5°K
P2 = ?
V2 = 3.3 L
T2 = -239°C = 34°K
Formula
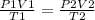
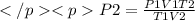
P2 = \frac{(3990)(3.41)(34)}{(181.5)(3.3)}[/tex]
P2 = 462600.6 / 598.95
P2 = 772.35 torr.